Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
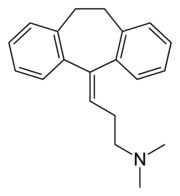
| 3-(10,11-dihydro-5H-dibenzo[a,d] cycloheptene-5-ylidene)-N, N-dimethyl-1-propanamine IUPAC name | |
| CAS number 549-18-8 |
ATC code |
| PubChem 2160 |
DrugBank APRD00227 |
| Chemical formula | {{{chemical_formula}}} |
| Molecular weight | 277.403 g/mol |
| Bioavailability | 40% |
| Metabolism | Hepatic |
| Elimination half-life | 12-24 hours |
| Excretion | Renal |
| Pregnancy category | C[1] |
| Legal status | |
| Routes of administration | Oral |
Amitriptyline (Tryptomer, Elavil, Tryptizol, Laroxyl, Saroten, Sarotex, Lentizol, Endep) is a tricyclic antidepressant (TCA). It is the most widely used TCA and has at least equal efficacy against depression as the newer class of SSRIs according to a study from early 2001.[2] As well as reducing depressive symptoms, these types of tricyclics also ease migraines, tension headaches, anxiety attacks and some schizophrenic symptoms. It is also known to reduce aggression and violent behaviour.
Uses[]
Approved[]
Amitriptyline is approved for the treatment of endogenous depression and involutional melancholia (depression of late life, which is no longer seen as a disease in its own right),[3] and reactive depression and for depression secondary to alcoholism and schizophrenia. Adult typical dosages are 75 to 200mg daily, with half this initially for elderly or adolescents. Amitriptyline is used for a number of medical conditions including: depressive disorders, anxiety disorders, attention deficit hyperactivity disorder, migraine prophylaxis, eating disorders, bipolar disorder, post-herpetic neuralgia, and insomnia.[4]
Amitriptyline is used in ankylosing spondylitis for pain relief. It is also used as a preventive for patients with recurring biliary dyskinesia (sphincter of Oddi dysfunction).[5]
Amitriptyline may be prescribed for other conditions such as cyclic vomiting syndrome post-traumatic stress disorder (PTSD),[6] chronic pain, tinnitus, chronic cough, carpal tunnel syndrome (CTS), fibromyalgia, vulvodynia, interstitial cystitis, male chronic pelvic pain syndrome, irritable bowel syndrome (IBS), diabetic peripheral neuropathy, neurological pain, laryngeal sensory neuropathy, chronic fatigue syndrome and painful paresthesias related to multiple sclerosis. Typically lower dosages are required for pain modification of 10 to 50 mg daily.[7]
A randomized controlled trial published in June 2005 found that amitriptyline was effective in functional dyspepsia that did not respond to a first-line treatment (famotidine or mosapride).[8]
It may also be used to treat nocturnal enuresis (bed wetting). Children between the ages of 7 to 10 years having a dose of 10 to 20 mg, older children 25 to 50mg at night. It should be gradually withdrawn at the end of the course, which overall should be of no more than 3 months.[7]
Unapproved/Off-Label/Investigational[]
Amitriptyline may be prescribed for other conditions such insomnia, migraine, rebound headache, chronic pain, postherpetic neuralgia (persistent pain following a shingles attack), fibromyalgia, vulvodynia, interstitial cystitis, irritable bowel syndrome and as a preventative (prophylaxis) for patients with frequent migraines. Typically lower dosages are required for pain modification of 10 to 50mg daily.[7]
Adverse effects[]
Template:Refimprove section The main two side effects that occur from taking amitriptyline are drowsiness and a dry mouth. Other common side effects of using amitriptyline are mostly due to its anticholinergic activity, including: weight gain, changes in appetite, muscle stiffness, nausea, constipation, nervousness, dizziness, blurred vision, urinary retention, and changes in sexual function. Some rare side effects include seizures, tinnitus, hypotension, mania, psychosis, sleep paralysis, hypnagogic or hypnopompic hallucinations related to sleep paralysis, heart block, arrhythmias, lip and mouth ulcers, extrapyramidal symptoms, depression, tingling pain or numbness in the feet or hands, yellowing of the eyes or skin, pain or difficulty passing urine, confusion, abnormal production of milk in females, breast enlargement in both males and females, fever with increased sweating, and suicidal thoughts.[9] The Indianapolis Discovery Network for Dementia (IDND) [10] rates Amitriptyline as having definite 'Anticholinergic Effects'. A side effect of many commonly used drugs with such effects appears to be to increase the risks of both cognitive impairment and death in older people, according to new research led by the University of East Anglia (UEA).[11] Amitriptyline can induce hepatotoxicity.[12]
Overdose[]
- Main article: Tricyclic antidepressant overdose
The symptoms and the treatment of an overdose are largely the same as for the other TCAs. The British National Formulary notes that Amitryptyline can be particularly dangerous in overdose,[13] thus it and other tricyclic antidepressants are no longer recommended as first line therapy for depression. Alternative agents, SSRIs and SNRIs are safer in overdose, though they are no more efficacious than TCAs.
Pharmacology[]
Amitriptyline acts primarily as a serotonin-norepinephrine reuptake inhibitor, with strong actions on the serotonin transporter and moderate effects on the norepinephrine transporter.[14][15] It has negligible influence on the dopamine transporter and therefore does not affect dopamine reuptake, being nearly 1,000 times weaker on it than on serotonin.[15]
Amitriptyline additionally functions as a 5-HT2A, 5-HT2C, 5-HT3, 5-HT6, 5-HT7, α1-adrenergic, H1, H2,[16] H4,[17][18] and mACh receptor antagonist, and σ1 receptor agonist.[19][20][21][22] It has also been shown to be a relatively weak NMDA receptor negative allosteric modulator at the same binding site as phencyclidine.[23] Amitriptyline inhibits sodium channels, L-type calcium channels, and Kv1.1, Kv7.2, and Kv7.3 voltage-gated potassium channels, and therefore acts as a sodium, calcium, and potassium channel blocker as well.[24][25][26]
Recently, amitriptyline has been demonstrated to act as an agonist of the TrkA and TrkB receptors.[27] It promotes the heterodimerization of these proteins in the absence of NGF and has potent neurotrophic activity both in-vivo and in-vitro in mouse models.[27][28] These are the same receptors BDNF activate, an endogenous neurotrophin with powerful antidepressant effects, and as such this property may contribute significantly to its therapeutic efficacy against depression. Amitriptyline does also act as FIASMA (functional inhibitor of acid sphingomyelinase).[29]
History[]
Amitriptyline, under the brand name Elavil, was developed by Merck and approved by the FDA on April 7, 1961, for the treatment of major depression in the United States.[30] In India, Merck & Co launched amitriptyline under the brand name Tryptomer, which is now with Merind, a division of Wockhardt.
See also[]
References[]
- ↑ Professional Information Brochure - ELAVIL®
- ↑ Barbui C, Hotopf M (February 2001). Amitriptyline v. the rest: still the leading antidepressant after 40 years of randomised controlled trials. The British Journal of Psychiatry : the Journal of Mental Science 178 (2): 129–144.
- ↑ Weissman MM. "The myth of involutional melancholia." Journal of the American Medical Association. 1979 Aug 24-31;242(8):742-4. PMID 459064
- ↑ Amitriptyline Hydrochloride. The American Society of Health-System Pharmacists. URL accessed on 3 April 2011.
- ↑ S. G. Hubscher et al. (2006). Functional biliary type pain syndrome. In P. J. Pasricha, W. D. Willis & G. F. Gebhart (Eds.), ' ' italics' ' Chronic Abdominal and Visceral Pain' 'italics' '. London: Informa Healthcare, pp. 459-461.
- ↑ National Institute for Clinical Excellence: The Treatment of PTSD in Adults and Children
- ↑ 7.0 7.1 7.2 British National Formulary 45 (March 2003). Cite error: Invalid
<ref>tag; name "BNF" defined multiple times with different content Cite error: Invalid<ref>tag; name "BNF" defined multiple times with different content - ↑ Otaka M (June 2005). New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment. Pharmacol. Ther. 21 (Suppl 2): 42–46.
- ↑ Healthline.com -Connect to Better Health
- ↑ http://www.indydiscoverynetwork.org/AnticholinergicCognitiveBurdenScale.html
- ↑ http://www.cfas.ac.uk/
- ↑ Drug-induced liver disease By Neil Kaplowitz, Laurie D. DeLeve; pag.527; http://books.google.com/books?id=ecgazhSpVX8C&pg=PA528&lpg=PA528&dq=Amitriptyline+hepatotoxic&source=bl&ots=MISv72vwjy&sig=3AwKzSeTEWjbqWKzlBSqeLjABHU&hl=en&ei=6XKkTseLA8SKswaF863gAg&sa=X&oi=book_result&ct=result&resnum=1&ved=0CBoQ6AEwADgK#v=onepage&q&f=false
- ↑ http://bnf.org/bnf/bnf/current/3295.htm
- ↑ https://archive.is/20130110184911/www.cnsforum.com/content/pictures/imagebank/hirespng/antidep_uptake_specific.png
- ↑ 15.0 15.1 PMID 9537821 (PMID 9537821)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ (1 January 1987) Progress in Medicinal Chemistry, Elsevier. URL accessed 27 November 2011.
- ↑ Nguyen T, Shapiro DA, George SR, et al. (March 2001). Discovery of a novel member of the histamine receptor family. Molecular Pharmacology 59 (3): 427–33.
- ↑ D. Sriram & P. Yogeeswari (1 September 2010). Medicinal Chemistry, Pearson Education India. URL accessed 27 November 2011.
- ↑ PMID 9400006 (PMID 9400006)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Alan F. Schatzberg, Charles B. (2006). Essentials of clinical psychopharmacology, American Psychiatric Pub.
- ↑ PMID 11561066 (PMID 11561066)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 17689532 (PMID 17689532)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 2568580 (PMID 2568580)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 9435180 (PMID 9435180)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ PMID 18048694 (PMID 18048694)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ Punke MA, Friederich P (May 2007). Amitriptyline is a potent blocker of human Kv1.1 and Kv7.2/7.3 channels. Anesthesia and Analgesia 104 (5): 1256–1264.
- ↑ 27.0 27.1 PMID 19549602 (PMID 19549602)
Citation will be completed automatically in a few minutes. Jump the queue or expand by hand - ↑ http://www.rxmed.com/b.main/b2.pharmaceutical/b2.1.monographs/CPS-%20Monographs/CPS-%20(General%20Monographs-%20A)/AMITRIPTYLINE.html
- ↑ Kornhuber J, Muehlbacher M, Trapp S, Pechmann S, Friedl A, Reichel M, Mühle C, Terfloth L, Groemer T, Spitzer G, Liedl K, Gulbins E, Tripal P (2011). Identification of novel functional inhibitors of acid sphingomyelinase. PLoS ONE 6 (8): e23852.
- ↑ Fangmann P, Assion HJ, Juckel G, González CA, López-Muñoz F (February 2008). Half a century of antidepressant drugs: on the clinical introduction of monoamine oxidase inhibitors, tricyclics, and tetracyclics. Part II: tricyclics and tetracyclics. Journal of Clinical Psychopharmacology 28 (1): 1–4.
External links[]
- PubChem Substance Summary: Amitriptyline National Center for Biotechnology Information.
- TREPILINE-10 TABLETS; TREPILINE-25 TABLETS South African Electronic Package Inserts. 12 May 1978. Revised February 2004.
- SAROTEN RETARD 25 mg Capsules; SAROTEN RETARD 50 mg Capsules South African Electronic Package Inserts. December 1987. Updated May 2000.
- AMITRIP Amitriptyline hydrochloride 10 mg, 25 mg and 50 mg Capsules Medsafe NZ Physician Data Sheet. November 2004.
- Endep Consumer Medicine Information, Australia. December 2005.
- MedlinePlus Drug Information: Amitriptyline. US National Institutes of Health. January 2008.
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |