Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
Monosaccharides are the simplest form of carbohydrates. They consist of one sugar and are usually colorless, water-soluble, crystalline solids. Some monosaccharides have a sweet taste. Examples of monosaccharides include glucose (dextrose), fructose, and galactose.
Monosaccharides are the building blocks of disaccharides like sucrose (common sugar) and polysaccharides (such as cellulose and starch). Further, each carbon atom that supports a hydroxyl group (except for the first and last) is chiral, giving rise to a number of isomeric forms all with the same chemical formula. For instance, galactose and glucose are both aldohexoses, but they have different chemical and physical properties.
Structure[]
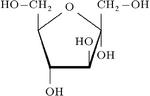
Fructose, a monosaccharide, as a Haworth projection.
With few exceptions (e.g. deoxyribose), monosaccharides have the empirical chemical formula (CH2O)n and the chemical structure H(CHOH)nC=O(CHOH)mH. If n or m is zero, it is an aldose, otherwise it is a ketose. Monosaccharides contain either a ketone or aldehyde functional group, and hydroxyl groups on most or all of the non-carbonyl carbon atoms.
Cyclic structure[]
Most monosaccharides form cyclic structures, which predominate in aqueous solution, by forming hemiacetals or hemiketals (depending on whether they are aldoses or ketoses) with themselves. Glucose, for example, forms a hemiacetal linkage between its carbon-1 and the hydroxyl group of its carbon-5. Since such a reaction introduces an additional chiral center, two anomers are formed from each distinct straight-chain monosaccharide. The interconversion between these two forms is called mutarotation.
A common way of representing the cyclic structure of monosaccharides is the Haworth projection.
Isomerism[]
The total number of possible stereoisomers of one compound (n) is dependent on the number of chiral centers (c) in the molecule: n = 2c.
Monosaccharide Nomenclature[]
Monosaccharides are classified by the number of carbon atoms they contain:
- Monose, 1 carbon atom
- Diose, 2 carbon atoms
- Triose, 3 carbon atoms
- Tetrose, 4 carbon atoms
- Pentose, 5 carbon atoms
- Hexose, 6 carbon atoms
- Heptose, 7 carbon atoms
- Octose, 8 carbon atoms
- Nonose, 9 carbon atoms
Monosaccharides are classified the type of keto group they contain:
Monosaccharides are classified according to their molecular configuration at carbon 2:
- D or d, configuration like in D-glyceraldehyde
- L or l, configuration like in L-glyceraldehyde
All these classifications can be combined, resulting in names like D-aldohexose or ketotriose.
List of monosaccharides[]
This is a list of some common monosaccharides, not all are found in nature - some have been synthesised:
- Monose: formaldehyde
- Diose: glycolaldehyde
- Trioses: glyceraldehyde and dihydroxyacetone
- Tetroses: erythrose threose
- Pentoses:
- Aldo-pentoses: arabinose, lyxose, ribose, deoxyribose, xylose
- Keto-pentoses: ribulose, xylulose
- Hexoses:
- Heptoses:
- Keto-heptoses: mannoheptulose, sedoheptulose
- Octoses: octolose, 2-keto-3-deoxy-manno-octonate
- Nonoses: sialose
Reactions[]
- Formation of acetals.
- Formation of hemiacetals and hemiketals.
- Formation of ketals.
See also[]
- Carbohydrate
- Disaccharide
- Oligosaccharide
- Polysaccharide
External links[]
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |