Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
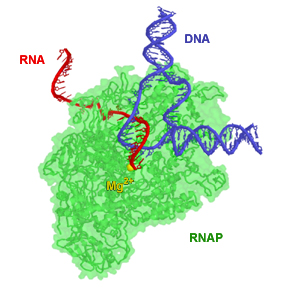
RNAP from E. coli pictured during elongation. Portions of the enzyme were made transparent so as to make the path of RNA and DNA more clear. The magnesium ion (yellow) is located at the enzyme active site
RNA polymerase (RNAP or RNApol) is an enzyme responsible for making RNA from a DNA template. RNAP accomplishes this task by constructing RNA chains through a process termed transcription. In scientific terms, RNAP is a nucleotidyl transferase that polymerizes ribonucleotides at the 3' end of an RNA transcript. RNA polymerase enzymes are essential and are found in all organisms, cells, and many viruses.
Control of transcription[]
Control of the process of transcription affects patterns of gene expression and thereby allows a cell to adapt to a changing environment, perform specialized roles within an organism, and maintain basic metabolic processes necessary for survival. Therefore, it is hardly surprising that the activity of RNAP is both complex and highly regulated. In E. coli bacteria, more than 100 factors have been identified which modify the activity of RNAP.
RNAP can initiate transcription at specific DNA sequences known as promoters. It then produces an RNA chain which is complementary to the DNA strand used as a template. The process of adding nucleotides to the RNA strand is known as elongation, and in eukaryotes RNAP can build chains as long as 2.4 million nucleosides (the full length of the dystrophin gene). RNAP will preferentially release its RNA transcript at specific DNA sequences encoded at the end of genes known as terminators.
Some RNA molecules produced by RNAP will serve as templates for the synthesis of proteins by the ribosome. Others can fold into enzymatically active ribozymes or tRNA molecules. A third option is that an RNA molecule will serve a purely regulatory role to control future gene expression (see siRNA).
RNAP accomplishes de novo synthesis. It is able to do this because specific interactions with the initiating nucleotide hold RNAP rigidly in place, facilitating chemical attack on the incoming nucleotide. Such specific interactions explain why RNAP prefers to start transcripts with ATP (followed by GTP, UTP, and then CTP). In contrast to DNA polymerase, RNAP includes a helicase activity, therefore no separate enzyme is needed to unwind DNA.
RNAP was discovered independently by Sam Weiss and Jerard Hurwitz in 1960. Ironically, by this time the 1959 Nobel Prize had been awarded to Severo Ochoa for the discovery of what was believed to be RNAP, but instead turned out to be a ribonuclease.
RNA polymerase in bacteria[]
In bacteria, the same enzyme catalyzes the synthesis of three types of RNA: mRNA, rRNA and tRNA.
RNAP is a relatively large molecule. The core enzyme has 5 subunits (~400 kDa):
- α2: the two α subunits assemble the enzyme and recognize regulatory factors.
- β: this has the polymerase activity (catalyzes the synthesis of RNA).
- β': binds to DNA (nonspecifically).
- ω: function not known clearly. However it has been observed to offer a protective/chaperone function to the β' subunit in M. smegmatis.
In order to bind promoter-specific regions, the core enzyme requires another subunit, sigma (σ). The sigma factor greatly reduces the affinity of RNAP for nonspecific DNA while increasing specificity for certain promoter regions, depending on the sigma factor. The complete holoenzyme therefore has 6 subunits: α2ββ'σω (~480 kDa). The structure of RNAP exhibits a groove with a length of 55 Å and a diameter of 25 Å. This groove fits well the 20 Å double strand of DNA. The 55 Å length can accept 16 nucleotides.
When not in use RNA polymerase binds to low affinity sites to allow rapid exchange for an active promotor site when one opens. RNA polymerase holoenzyme, therefore, does not freely float around in the cell when not in use.
RNA polymerase in eukaryotes[]
Eukaryotes have several types of RNAP:
- RNA polymerase I synthesizes a pre-rRNA 45S, which matures into 28 S, 18S and 5,8S rRNAs which will form the major RNA sections of the ribosome.
- RNA polymerase II synthesizes precursors of mRNAs and most snRNA. This is the most studied type, and due to the high level of control required over transcription a range of transcription factors are required for its binding to promoters. For detail of RNA polymerase function please see RNA polymerase II.
- RNA polymerase III synthesizes tRNAs, rRNA 5S and other small RNAs found in the nucleus and cytosol.
- Other RNA polymerase types in mitochondria and chloroplasts.
RNA polymerase in archaea[]
Archaea have a single form of RNAP that is closely related to the three main eukaryotic polymerases. It has been speculated that the archaeal polymerase resembles the ancestor of the specialized eukaryotic polymerases.
RNA polymerase in viruses[]
Many viruses also encode for RNAP. The viral polymerases are diverse, and include some forms which can use RNA as a template instead of DNA (this occurs in polio). Perhaps the most widely studied viral RNAP is found in bacteriophage T7. This single-subunit RNAP is related to that found in mitochondria and chloroplasts, and shares considerable homology to DNA polymerase. It is believed by many that most viral polymerases therefore evolved from DNA polymerase and are not directly related to the multi-subunit polymerases described above.
Transcriptional cofactors[]
There are a number of proteins which can bind to RNAP and modify its behavior. For instance, greA and greB from E. coli can enhance the ability of RNAP to cleave the RNA template near the growing end of the chain. This cleavage can rescue a stalled polymerase molecule, and is likely involved in proofreading the occasional mistakes made by RNAP. A separate cofactor, Mfd, is involved in transcription-coupled repair, the process in which RNAP recognizes damaged bases in the DNA template and recruits enzymes to restore the DNA. Other cofactors are known to play regulatory roles, i.e. they help RNAP choose whether or not to express certain genes.
Isolation[]
RNA polymerase can be isolated in the following ways:
- By a phosphocellulose column (PC).
- By glycerol gradient centrifugation (GG).
- By a DNA column (A).
- By an Ion exchange column.
- PC+A
See also[]
- DNA polymerase
- T7 RNA polymerase
External links[]
- DNAi - DNA Interactive, including information and Flash clips on RNA Polymerase.
de:RNA-Polymerase es:ARN polimerasa fr:ARN polymérase he:RNA פולימראז nl:RNA-polymerase pt:ARN-polimerase vi:RNA polymerase
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |