Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
- "Reduced" redirects here. For other senses of that word, see reduction.
Semi-accurate illustration of a redox reaction
Redox reactions include all chemical processes in which atoms have their oxidation number (oxidation state) changed.
This can be a simple redox process, such as the oxidation of carbon to yield carbon dioxide, it could be the reduction of carbon by hydrogen to yield methane (CH4), or it could be the oxidation of sugar in the human body, through a series of very complex electron transfer processes.
The term redox comes from the two concepts of reduction and oxidation. It can be explained in simple terms:
- Oxidation describes the loss of an electron by a molecule, atom or ion
- Reduction describes the gain of an electron by a molecule, atom or ion
However, these descriptions (though sufficient for many purposes) are not truly correct. Oxidation and reduction properly refer to a change in oxidation number — the actual transfer of electrons may never occur. Thus, oxidation is better defined as an increase in oxidation number, and reduction as a decrease in oxidation number. In practice, the transfer of electrons will always cause a change in oxidation number, but there are many reactions which are classed as "redox", though no electrons are transferred (such as those involving covalent bonds).

The Two Parts of a Redox Reaction.

The rusting of iron.

A bonfire. Combustion consists of redox reactions involving free radicals.
Oxidizing and reducing agents[]
Substances that have the ability to oxidize other substances are said to be oxidative and are known as oxidizing agents, oxidants or oxidizers. Put in another way, the oxidant removes electrons from the other substance, and is thus reduced itself. Oxidants are usually chemical substances with elements in high oxidation numbers (e.g., H2O2, MnO4-, CrO3, Cr2O72-, OsO4) or highly electronegative substances that can gain one or two extra electrons by oxidizing a substance (O, F, Cl, Br).
Substances that have the ability to reduce other substances are said to be reductive and are known as reducing agents, reductants, or reducers. Put in another way, the reductant transfers electrons to the substance. Reductants in chemistry are very diverse. Metal reduction - electropositive elemental metals can be used (Li, Na, Mg, Fe, Zn, Al). These metals donate or give away electrons readily. Other kinds of reductants are hydride transfer reagents (NaBH4, LiAlH4), these reagents are widely used in organic chemistry, primarily in the reduction of carbonyl compounds to alcohols. Another useful method is reductions involving hydrogen gas (H2) with a palladium, platinum, or nickel catalyst. These catalytic reductions are primarily used in the reduction of carbon-carbon double or triple bonds.
The chemical way to look at redox processes is that the reductant transfers electrons to the oxidant. Thus, in the reaction, the reductant or reducing agent loses electrons and is oxidized and the oxidant or oxidizing agent gains electrons and is reduced.
Examples of redox reactions[]
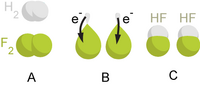
A good example is the reaction between hydrogen and fluorine:
We can write this overall reaction as two half-reactions: the oxidation reaction
and the reduction reaction:
Analysing each half-reaction in isolation can often make the overall chemical process clearer. Because there is no net change in charge during a redox reaction, the number of electrons in excess in the oxidation reaction must equal the number consumed by the reduction reaction (as shown above).
Elements, even in molecular form, always have an oxidation number of zero. In the first half reaction hydrogen is oxidized from an oxidation number of zero to an oxidation number of +1. In the second half reaction fluorine is reduced from an oxidation number of zero to an oxidation number of −1.
When adding the reactions together the electrons cancel:
And the ions combine to form hydrogen fluoride:
Other examples[]
- iron(II) oxidizes to iron(III):
- Fe2+ → Fe3+ + e-
- hydrogen peroxide reduces to hydroxide in the presence of an acid:
- H2O2 + 2 e- → 2 OH-
overall equation for the above:
- 2Fe2+ + H2O2 + 2H+ → 2Fe3+ + 2H2O
- denitrification, nitrate reduces to nitrogen in the presence of an acid:
- 2NO3- + 10e- + 12 H+ → N2 + 6H2O
- iron oxidizes to iron(III) oxide and oxygen is reduced forming iron(III) oxide (commonly known as rusting or tarnishing):
- 4Fe + 3O2 → 2 Fe2O3.
- Combustion of hydrocarbons, e.g. in an internal combustion engine, produces water, carbon dioxide, some partially oxidized forms such as carbon monoxide and heat energy. Complete oxidation of materials containing carbon produces carbon dioxide.
- In organic chemistry, stepwise oxidation of a hydrocarbon produces water and, successively, an alcohol, an aldehyde or a ketone, carboxylic acid, and then a peroxide.
Redox reactions in biology[]
Need neurochemistry examples
Much biological energy is stored and released by means of redox reactions. Photosynthesis involves the reduction of carbon dioxide into sugars and the oxidation of water into molecular oxygen. The reverse reaction, respiration, oxidizes sugars to produce carbon dioxide and water. As intermediate steps, the reduced carbon compounds are used to reduce nicotinamide adenine dinucleotide (NAD+), which then contributes to the creation of a proton gradient, which drives the synthesis of adenosine triphosphate (ATP) and is maintained by the reduction of oxygen. In animal cells, mitochondria perform similar functions. See Membrane potential article.
The term redox state is often used to describe the balance of NAD+/NADH and NADP+/NADPH in a biological system such as a cell or organ. The redox state is reflected in the balance of several sets of metabolites (e.g., lactate and pyruvate, beta-hydroxybutyrate and acetoacetate) whose interconversion is dependent on these ratios. An abnormal redox state can develop in a variety of deleterious situations, such as hypoxia, shock, and sepsis. Redox signaling involves the control of cellular processes by redox processes.
Mnemonics[]
The key terms involved in redox can be confusing. For example, an element that is oxidized loses electrons; however, that element is referred to as the reducing agent. Likewise, an element that is reduced gains electrons and is referred to as the oxidizing agent. Several acronyms and mnemonics are often used to remember what is happening:
- "OIL RIG"—Oxidation Is Loss, Reduction Is Gain.
- "LEO the lion says GER"—Losing Electrons is Oxidation, Gaining Electrons is Reduction.
- Visualize OXen going up a mountain—just as the oxidation number of an OXidation goes up—and RED blood flowing down the mountain—just as the oxidation number of a REDuction goes down.
- "EOH" remind you to include an electron and oxygen and hydrogen atom(s) in your complex equations.
Redox Cycling[]
A wide variety of aromatic compounds are enzymatically reduced to form free radicals that contain one more electron than their parent compounds. In general, the electron donor is any of a wide variety of flavoenzymes and their coenzymes. Once formed, these anion free radicals reduce molecular oxygen to superoxide and regenerate the unchanged parent compound. The net reaction is the oxidation of the flavoenzyme's coenzymes and the reduction of molecular oxygen to form superoxide. This catalytic behavior has been described as futile cycle or redox cycling. Examples of redox cycling-inducing molecules are the herbicide paraquat and other viologens and quinones such as menadione.
References[]
See also[]
- Bioremediation
- Calvin cycle
- Citric acid cycle
- Electrochemical cell
- Electrochemistry
- Galvanic cell
- Membrane potential
- Thermic reaction
- Reducing agent
External links[]
- Redox Reactions Calculator
- Redox reactions at Chemguide
- Online Redox Reaction Equation Balancer balances equations of any half-cell and full reactions
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |






